Dr. Adinarayana Doddi Assistant Professor, Department of Chemical Sciences IISER Berhampur
Elusive Main Group Radicals and Cations: Carbene Supported Arsenic Monophosphide [AsP] and Its Radical Cation and Dication
In 1991, a stable and colorless crystalline diaminocarbene, IAd (1,3-di-1-adamantyl-imidazol-2-ylidene) was reported first time by Arduengo and co-workers.[1] After its discovery, N-heterocyclic carbenes (NHCs) have become alternative ligands to the, until then established, phosphine ligands in the transition metal coordination chemistry. They have found numerous applications in diverse fields due to their unique electronic character as they are not only good sigmadonors but also weak pi-acceptors. [2,3] These properties have played crucial role especially in the stabilization of novel and unusual molecular organometallic fragments, development of new homogeneous catalysts (as ancillary ligands). Carbene stabilization of elusive diatomic molecules composed of lighter p-block elements has been one of the most landmark discoveries and very elegant applications of N-heterocyclic carbenes.[4] Similar to the homo-dinucleardiphosphorus (P2 ), diarsenic (As2 ) molecules of the group 15 elements, the heterodiatomic “Arsenic Monophosphide (AsP)” does exist which was studied spectroscopically in the gas phase and in the solid Neon matrix. Interestingly, the dissociation energy (429.7 kJ mol-1) of “AsP” molecule, determined by the mass spectroscopic analysis indicates that this value is approximately the average bond dissociation energies of the individual homodiatomic components As2 (379.1 kJ mol-1) and P2 (485.8 kJ mol-1). It should be noted that the unsupported “AsP” moiety as a ligand has so far only been stabilized in the coordination sphere of transition metal complexes,[5] however, never been isolated as a free form.
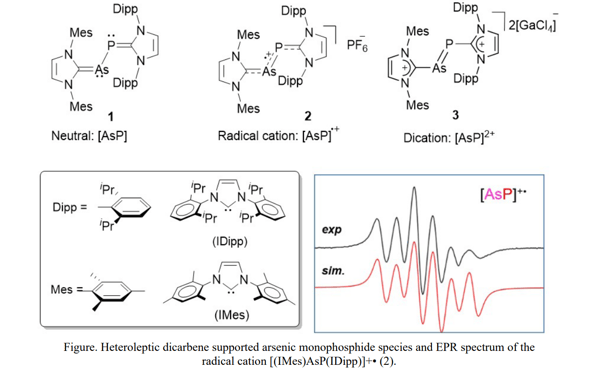
A.Doddi and M. Tamm et al.[6] developed a novel synthetic procedure for the isolation of a series of heterolepticdicarbene supported arsenic monophosphide [AsP] species of the type [(IMes)AsP(IDipp)]n (n = 0 (1), 1 (2), 2 (3)). Compounds 1-3were prepared by a modular approach involving stepwise reactions byconnecting two different centers of arsenic and phosphoruswhich are already supported by two different N-heterocyclic carbenes. Reduction of the mono cationic dichloro derivative[(IMes)As(Cl)P(IDipp)]Cl with two equivalents of the reducing agent, potassium graphite (KC8 ) in THF afforded the neutral arsenic monophosphide[(IMes)AsP(IDipp)] (1), and the subsequent one electron oxidation reaction with ferrocenium hexafluorophosphate ([Fc]PF6 ) afforded the radical cation [(IMes)AsP(IDipp)]PF6 (2).The dicationic species [(IMes)AsP(IDipp)]2+(3) was also achieved by formally two electron oxidation or the abstraction of both chlorides from the dichloro derivative [(IMes)As(Cl)P(IDipp)]Cl.
Solid state molecular structures of 1–3 have been established by single crystal X-ray diffraction analysis. Broad singlet resonances observed in the 31P NMR spectra of the neutral (1, –60.6 ppm) and dicationic (3, 475.5 ppm) species indicate the presence of an arsenic-phosphorus (As–P) single bond in the neutral species [(IMes)AsP(IDipp)] (1) and double bond (As=P) in the dication [(IMes)AsP(IDipp)]2+. Whereas, the radical character of the mono cation [(IMes)AsP(IDipp)]+•(2)was confirmed by EPR (Electron Paramagnetic Resonance) spectroscopy.As depicted in the Figure, the X band EPR spectrum at room temperature showed a “pseudo-sextet” pattern withan isotopic g value of 2.0246 and hyperfine coupling to 75As (I = 3/2) and 31P (I = 1/2) nuclei.It should be noted that a very similar pattern was also reported for the arsaphosphene radical anion [RAsPR]–• (R = sterically bulky aryl group) which can be considered as a closely related arsenic-phosphorus main group radical system. [7]Complete details of the published work can be accessed from the reference no.6.
References:
1. A. J. Arduengo, R. L. Harlow and M. Kline, J. Am. Chem. Soc.1991, 113,361–363.
2. A. Doddi, M. Peters, M. Tamm, Chem. Rev.2019, 119, 6994–7112.
3. V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt, S. Inoue, Chem. Rev.2018, 118, 9678–9842.
4. a) Y. Wang, G. H. Robinson, Inorg. Chem.2011, 50, 12326–12337; b) Y. Wang, G. H. Robinson, Dalton Trans.2012, 41, 337–345;
5. J. E. Davies, L. C. Kerr, M. J. Mays, P. R. Raithby, P. K. Tompkin, A. D. Woods, Angew. Chem. 1998, 110, 1473–1475; Angew. Chem. Int. Ed.1998, 37, 1428–1429.
6. A. Doddi, D. Bockfeld, T. Bannenberg, M. K. Zaretzke and M. Tamm. Chem. Eur. J. 2019, 00, 00-00 (accepted; DOI.10.1002/ chem.201903795).
7. K. Tsuji, Y. Fujii, S. Sasaki, M. Yoshifuji, Chem. Lett.1997, 26, 855–856.