
A View of Metal-Based Drugs in Cancer Treatment
Kumar Gaurav, 3rd Year BS-MS, Dept. of Chemical Sciences, IISER Berhampur
Cancers are a huge family of diseases that involve abnormal growth of cells with a high potential of spreading to other parts of the body. Among several diseases, cancer has become a big challenge for human beings and is the second most common cause of death all over the world, responsible for high mortality after cardiovascular disease. Due to this disease, an average of 0.3 million deaths have been documented.
[1] The majority of cancers, 90-95% of cases are due to the mutations from environmental factors and the remaining 5-10% is of inherited genetics. In general, it is not possible to prove the cause of this disease as many factors play a role.[2] They don’t have any specific fingerprint, for example, if a person uses tobacco heavily, he/she might develop lung cancer, and then most probably it is due to the heavy usage of tobacco or related products. It can’t be ignored that lung cancer can also develop due to radiation and air pollution. Furthermore, other reasons for this disease include the rare transmissions that occur with pregnancies and occasionally organ donors. However, and unlike many other diseases, cancer is not a transmissible disease. Globally, approx.18% of deaths are due to cancer, and up to 10% of invasive cancers are related to exposure including both ionizing radiation and non-ionizing ultraviolet radiation. There are more than 100 types of cancers have been detected, such as breast cancer, skin cancer, lung cancer, colon cancer, prostate cancer, and lymphoma, etc. It can further be categorized into carcinoma, sarcoma, lymphoma and myeloma, and central nervous system cancers. Although several methods of treating different cancers have been developed, however, chemotherapy, radiation, and surgery are the most widely employed and notable methods of treatment in cancer therapy. The main aim of this monograph is to inform the reader about the current state of the art with respect to the value of a wide spectrum of metal complexes in cancer therapy.
Over the last few years, many scientists have been working worldwide on the development of new, efficient, cheap anticancer agents and this has been the frontier research in recent times. Within this realm of cancer research, biological inorganic chemistry (bio-inorganic chemistry) has shown its importance to help cancer treatment and played a vital role. This is perhaps due to the fact that many transition metals have unique features and are capable of exhibiting variable coordination modes coupled with redox activities, and moderate to high reactivity towards various organic molecules in the biochemical process. As stated above, metal complexes from the d-block of the periodic table of elements have been widely employed in the treatment of various cancers by chemotherapy and it has been found that these metals play an effective positive role in the healing of various cancer cells. The most famous anticancer drug “Cisplatin” was discovered by Barnett Rosenberg in 1960. It can be considered as a milestone in the history of bio-inorganic chemistry and it has witnessed the potential and importance of metals in the discipline of medicinal chemistry. Since its discovery, several platinum-based platinum-based metal complexes have been designed and used for the treatment of different cancers. The most notable and commercially available (see Figure 1) anticancer drugs are cisplatin, oxaliplatin, and carboplatin. In addition to these complexes, there were many more metal-based complex compounds synthesized by redesigning the existing chemical structures through the ligand substitution or building up of entirely new compounds with the enhancement of safety and cytotoxicity. By viewing several articles in the literature,[3] it should be noted that numerous metal complexes including platinum, ruthenium, rhodium, iron also found to be active against various cancers which have several specified applications such as conjugation to DNA and RNA, cell penetration and bio-sensing.
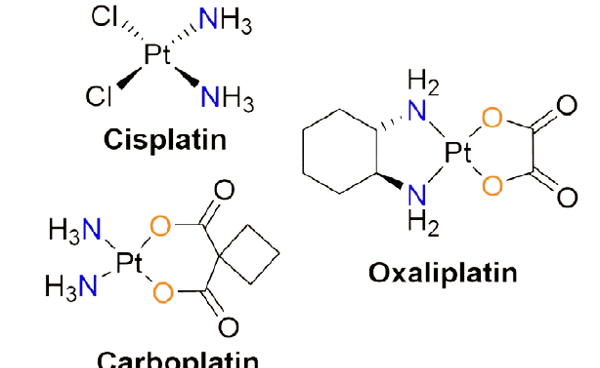
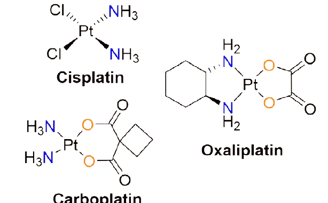
Figure 1. Selected examples of platinum-based anticancer agents which are commercially available.
As mentioned above, metals are very essential components of cells in nature to function in many important biochemical processes for living organisms. They are involved in multiple biological processes and are frequently found in the enzyme catalytic domain. Various transition metals such as zinc, cobalt, gallium, vanadium, silver, strontium, manganese, and copper are required in trace amounts to elicit catalytic processes.[3] It is important for the normal physiological state to balance between cellular needs and the amount available in the body. Some metals including cadmium,[4] chromium, nickel, and arsenic, can induce carcinogenesis and therefore are less useful to the body. It is interesting to note that molecular studies and clinical trials have suggested some arsenic derivatives such as arsenic trioxide could serve as chemotherapeutic agents in the treatment of malignant tumors.[5] Therefore, such limitations have prompted several research groups to search for other alternative metals and their complexes. In this context, an intense search was undertaken for the platinum-based compounds that show lesser toxicity, greater selectivity, and a broad range of activity.
The platinum (II) complexes such as oxaliplatin, carboplatin as well as other platinum derivatives are the products of this search. In addition to the former platinum-based complexes, other metal compounds consisting of chelating ligands coordinated to the metal centers such as zinc, gold, and copper are playing an essential role as anticancer agents. [3] Figure 2 shows a collection of several organometallic complexes employed in cancer research.
The utility of other late transition metals such as ruthenium and gold-based compounds has recently received attention as new anticancer agents due to their favorable cytotoxic and potential anticancer activity.[3] Moreover, ligand substitution and modification of existing chemical structures led to the synthesis of a wide variety of metal-based compounds, some of which have demonstrated enhanced pharmacokineticity, safety, and cytotoxicity. It can be noted that about 50% of all the cancer treatments by chemotherapy, mainly utilizing platinum-based anticancer drugs namely, cisplatin, carboplatin, oxaliplatin, nedaplatin, lobaplatin, and heptaplatin. Several reports revealed that these drugs show side effects along with tumor resistance to the drugs. In order to reduce as many as possible side effects and toxicity to the other healthy cells, new compounds need to be designed which show less toxic and more tumor selectivity, and the enhancement of their potential and activity toward cancer cells. Along with this line, other metal-based complexes, alternatives to the well-established Pt-based drugs are developed, such as the ruthenium (NAMI-A, KP1019, NKP1339), rhodium (dirhodiumtetraacetate), gold, and iron-based complexes. In addition to the aforementioned expensive metals, iron metal-based complexes have also been investigated as anticancer agents owing to their unique biological connection and as essentiality in numerous biological processes. It has been reported that upon incorporation into the target proteins, iron participates in a variety of cellular biochemical reactions, such as electron transport, DNA synthesis, and erythropoiesis. [6] Iron exists in two common oxidation states, the ferrous [Fe(II)] and ferric [Fe(III)], which makes it undergo a transformation from one to the other. The donation or acceptance of an electron enables it to carry out a wide range of biological functions. In summary, it is evident that not only in nature but also many biological processes make use of metals in their life cycle and thus the medicinal activity of several transition metals triggered the development of a plethora of metal-based anticancer agents consisting of various organic ligands. It is noteworthy to mention that toxic metals such as cadmium and arsenic-containing complexes have also proved to be active agents in healing different cancers. Nevertheless, the cisplatin and related platinum complexes have been in the mainstream of cancer therapy, some cancers have proved to be resistant, thus, an extensive search for the design of alternative metal complexes can be explored which might show improved anticancer activity. It can be envisioned that the potential of various iron-based complexes can be studied as they have already attracted attention from the scientific community. Molecular studies and clinical trials of several main groups and transition metal-based complexes suggest that they can be potential anticancer agents and hopefully a good number of them will be practically useful for treating different cancers in the near future. It is a known fact that many commercially available anticancer drugs containing platinum metals are expensive. Therefore, earth-abundant, user-friendly and non-toxic metals-based metallo-anticancer drugs can still be developed, in doing so, they can be cheap and affordable for common people.
*[1] I. Ali, W. A. Wani and K. Saleem., Cancer Therapy, 2011, 8, 56-70. [2] P. Anand, A. B. Kunnumakara, C. Sundaram, K.B. Harikumar, S. T. Tharakan, O. S. Lai, B. Sung, and B. B. Aggarwal., Pharm Res., 2008, 9, 2097-2116. [3] U. Ndagi, N. N. Mhlongo and M. E Soliman., Drug Design, Development and Therapy, 2017, 11, 599-616. [4] Asara et al., Eur. J. Histochem., 2012, 56, e1. [5] S. Waxman, K. C. Anderson, History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001, 6(suppl 2), 3-10. [6] W. A. Wani , U. Baig, S. Shreaz, R. A. Shiekh, P. F. Iqbal, E. Jameel, A. Ahmad, S. H. Mohd-Setapar, Md. Mushtaqueh and L. T. Hun., New J. Chem., 2016, 40, 1063-1090.
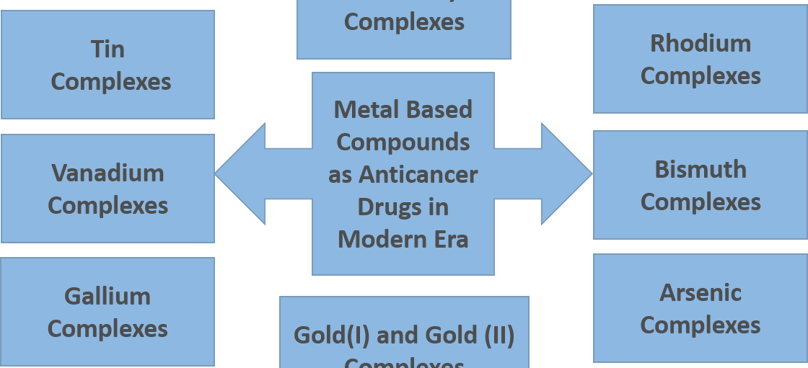
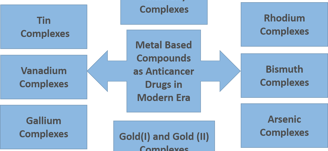
Figure 2. A schematic representation of metal (organometallic) complexes in cancer therapy other than platinum complexes


