
Prof. K.V.R. Chary, Director, IISER Berhampur
Insights Into Congenital Cataract
A recently identified G57W mutant of human γScrystallin is associated with dominant congenital cataracts, the familial determinate of childhood blindness worldwide[1] [2]. To investigate the structural and functional changes that mediate the effect of this cataract-related mutant to compromise eye lens transparency and cause lens opacification in children, we optimized the expression and purification of [u-15N, 13C] γS-G57W in high yields for NMR studies[2] and recently reported its complete sequences pecific resonance assignments using a suite of multinuclear multidimensional NMR experiments [3]. With this in the backdrop, we determined the high resolution 3D structure of γS-G57W (see Figure) which is recalcitrant towards crystallization and studied its conformational dynamics by solution NMR spectroscopy. Our structural inference that the unusual solvent exposure of W57 is associated with rearrangement of the N-terminal domain suggest an efficient pathway for increased aggregation in γS-G57W and illuminates the molecular dynamics underlying cataractogenic aggregation of lens crystallins in particular and aggregation of proteins in general[4]. Further, we reported residue resolved conformational dynamics in both γS-WT and γS-G57W for the first time using solution NMR spectroscopy, and suggest how these differences could crucially affect the biochemistry of the mutant. Guided by our critical structural investigations, extensive conformational dynamics and biophysical studies, we showed that loss of structural stability arises from enhanced dynamics in Greek key motif 2 inducing flexibility in the N-terminal domain as opposed to its structurally unperturbed C-terminal counterpart [5]. Our results highlight the vital role of conserved Greek key motifs in conferring structural stability to crystallins and provide crucial molecular insights into crystallin aggregation in the eye lens, which triggers cataract formation in children.
References
[1] Bari KJ, Sharma S and Chary KVR. Sequence specific 1H, 13C and 15N resonance assignments of the C-terminal domain of human γS-crystallin. Biomol. NMR Assign., 2019, 13(1) 43-47.
[2] Bari KJ, Sharma S and Chary KVR. Structural and functional characterization of a missense mutant of human γS-crystallin associated with dominant infantile cataracts. Biochem Biophys Res Commun., 2018. 506(4) 862-867.
[3] Bari KJ, Sharma S and Chary KVR. Sequence specific 1H, 13C and 15N resonance assignments of a cataract-related variant G57W of human γS-crystallin. Biomol. NMR Assign., 2018, 12(1) 51-55.
[4] Bari KJ, Sharma S and Chary KVR. Structure of G57W mutant of human γS-crystallin and its involvement in cataract formation. J Struct Biol., 2019, 205(3) 72-78.
[5] Bari KJ, Sharma S and Chary KVR. Conformational dynamics study on human γScrystallin as an efficient route to childhood blindness. Biochem Biophys Res Commun., 2019, 511(3) 679-684.
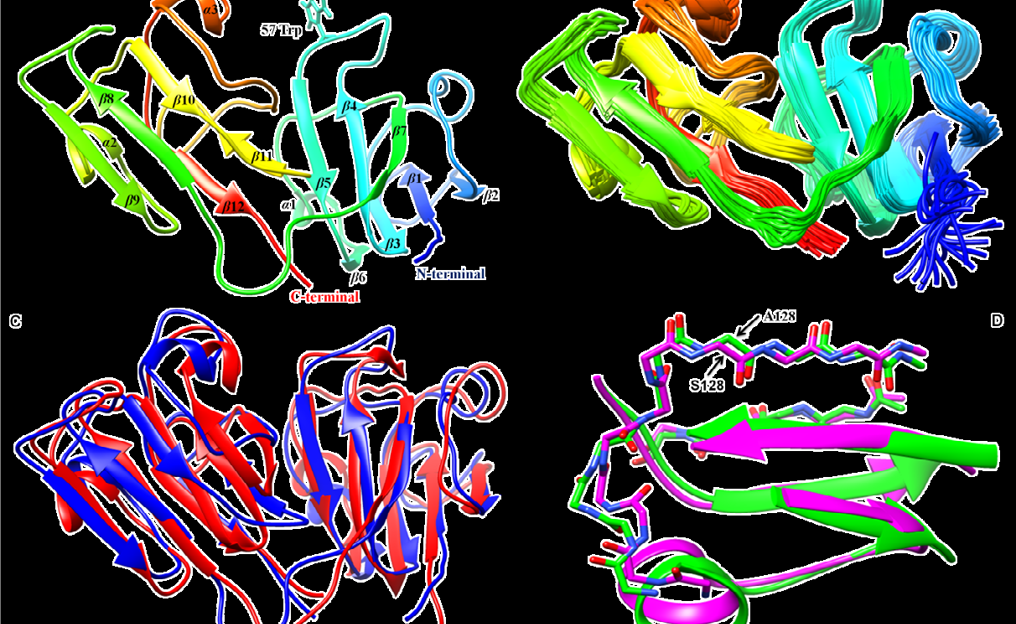
Figure: Structural details of human γS-G57W. (A) The energy minimized solution NMR structure of full length γS-G57W calculated using CYANA. The Trp at the 57th position (W57), the site of mutation is seen exposed to the solvent. (B) An ensemble of 20 superimposed minimum energy NMR-derived conformers of γS-G57W. (C) An overlay of the NMR structure of γS-G57W (PDB: 6IF9) (shown in blue) and chicken-γS crystallin (PDB: 5VH1) (shown in red) shows changes at the mutated loop region. (D) Structural alignment of residues around position S128 between the two structures. Chicken γS-crystallin contains a non-conserved A128 instead of Ser (This is 129th residue in human γS-G57W as it starts with M1) with no change in its local conformation.


